Herbal Monograph Preparation
- Introduction
Pharmacopoeial monographs for herbal medicines should contain information in the definition that is consistent with the monograph title, followed by specifications for quality including identity, purity and content. Individual monographs describe test procedures, together with the corresponding specifications. The monograph may include:
■ An official title;
■ A definition;
■ A production section;
■ An identification section;
■ A test section covering, for example, physicochemical tests and, where appropriate, tests on contaminants;
■ An assay section on determining constituents with known therapeutic activity, active or analytical markers.
2. Importance
Herbal monographs in national pharmacopoeias and other authoritative documents play an important role in the authentication of herbal materials. In this context, a monograph is a document that defines a botanical drug and provides information that allows for its proper identification.
In Australia, herbal raw materials are required to be authenticated to the relevant monograph in the British Pharmacopoeia, if such a monograph exists. Other official pharmacopoeias, such as the European Pharmacopoeia, the Pharmacopoeia of China, the United States Pharmacopoeia and National Formulary, and the Indian Ayurvedic Pharmacopoeia, contain monographs for many medicinal plant species not included in the British Pharmacopoeia.
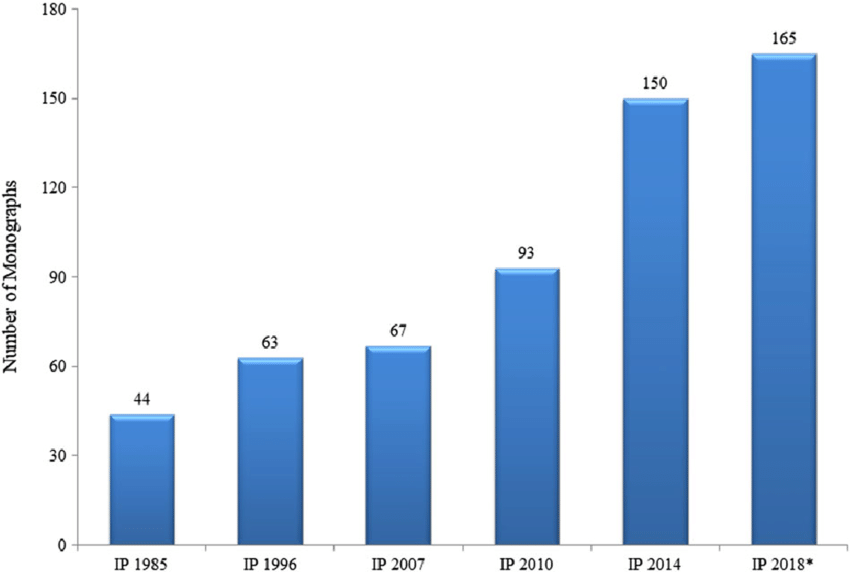
Pharmacopoeial monographs for medicinal plants often describe several methods of identification. A herbal monograph in the British Pharmacopoeia, for example, will typically include a macroscopic description, a microscopic description, and a thin-layer chromatography test. The Indian Pharmacopoeia (Edition 2018) contains monographs for 165 of medicinal plants used in Ayurvedic Medicine.
Regulation And Guidelines
In India there are laws dealing with drugs that are the subject of monographs which follow. These monographs should be read subject to the restrictions imposed by these laws wherever they are applicable.
It is expedient that enquiry be made in each case in order to ensure that the provisions of the law are being complied with.
In general, the Drugs & Cosmetics Act, 1940 (subsequently amended in 1964 and 1982), the Dangerous Drugs Act, 1930 and the Poisons Act, 1919 and the rules framed thereunder should be consulted. Under the Drugs & Cosmetics Act, the Ayurvedic Pharmacopoeia of India (A.P.I.), Part-I, Vol. IV, is the book of standards for single drugs included therein and the standards prescribed in the Ayurvedic Pharmacopoeia of India, Part-I, Vol. IV would be official. If considered necessary these standards can be amended and the Chairman of the Ayurvedic Pharmacopoeia Committee authorized to issue such amendments. Whenever such amendments are issued the Ayurvedic Pharmacopoeia of India, Part-I, Vol. IV, would be deemed to have been amended accordingly.[1]
HISTORY OF HERBAL DRUGS STANDARDS IN THE INDIAN PHARMACOPOEIA
The IP is a legally recognized book of standards for drugs and their formulations in India. The standards of identity, purity and strength prescribed in IP are to ensure quality of the medicines (Garg, 2016). IP is published by the IPC, Ministry of Health and Family Welfare, Government of India. Addendum/Addenda to the main version of IP is/are published from time to time to takecare of urgent requirements of changes in the existing monographs and for inclusion of new monographs. The Addendum has the same authority as IP.
The origin of IP goes back to the publication of the Bengal Pharmacopoeia and General Conspectus of Medicinal Plants 1844 generally known as Bengal Pharmacopoeia. The focus of this pharmacopoeia was on indigenous drugs although it included some products imported from Europe. The first pharmacopoeia of India was published in 1868, which contained drugs official in the British Pharmacopoeia 1867 and some indigenous drugs. The Indian Pharmacopoeial List 1946 was prepared to serve as an Indian supplement to the British Pharmacopoeia 1932.
After independence, an Indian Pharmacopoeia Committee was constituted in 1948, which prepared the Pharmacopoeia of India (The Indian Pharmacopoeia) in 1955. A supplement to it was published in 1960. This pharmacopoeia contained western and also traditional drugs, and the same policy continued while preparing the Pharmacopoeia of India: The Indian Pharmacopoeia, 1966 and its 1975 supplement. In the Pharmacopoeia of India, 1985, and its Addenda, 1989 and 1991, traditional drugs were not included, as publication of a pharmacopoeia of traditional system drugs was taken up separately, and only those herbal drugs were included that had supporting definitive quality control standards (Indian Pharmacopoeia, 1996a).
In IP 1966, an initial start regarding the vegetable drugs standards was made in respect of 10 drugs that were in wide use at that time. The authentic samples of these drugs were obtained and investigated at different laboratories in the country. On the basis of the results of these investigations, the Indian Medicinal Plants Sub-Committee drew up standards of the following three drugs: Jatamanshi (Nardostachys jatamansi), Rasna (Alpinia officinarum) and Vidang (Embelia ribes) that were then included as monographs. Preparations of
these drugs, however, had not been included in this edition as standards because of non-availability of those preparations (Indian Pharmacopoeia, 1996a). Indian Pharmacopoeia, Addendum 2005 (1996b) introduced 10 new drugs including Ashwagandha, Bacopa, Bhuiamla, Centella, Garcinia, Ginger, Kalmegh, Sallaki, Turmeric and Vasaka.
Indian Pharmacopoeia (2007a) incorporated for the first time a chapter on the general requirements of herbs and herbal products standards and a total of 58 specific monographs, including 23 new monographs. The new monographs included were Amalaki, Amra, Arjuna, Artemisia, Bhibhitaki, Bhringraj, Coleus, Gokhru, Gudmar, Guduchi, Haritaki, Kunduru, Kutki, Lasuna, Manjistha, Maricha, Pippali Large, Pippali Small, Punarnava, Sarpagandha, Shatavari, Shati and Tulasi. Indian Pharmacopoeia, Addendum 2008 (2007b)
incorporated nine new monographs, namely, Ajwain, Anantmula, Daruharidra Roots, Daruharidra Stems, Kalmegh Dry Extract, Saunf, Senna Dry Extract, Senna Tablets and Yasti Dry Extract.
Indian Pharmacopoeia (2010a) contained 89 specific monographs of herbs and herbal products and its Addendum 2012 (Indian Pharmacopoeia, 2010b) incorporated four new monographs, namely, Bhuiamla Dry Extract, Gudmar Dry Extract, Kunduru Dry Extract and Mandukaparani Dry Extract with a total figure of 93 monographs.[12]
CURRENT STATUS OF HERBAL DRUGS STANDARDS IN IP
The eight edition of IP (2018) containing four volumes came into effect from 1 April 2018. Herbal drugs standards are covered in Volume III.
New Monographs : 220
Revisions : 366
Omissions : 07
170 New Chemical Monographs :
49 API
64 Formulations
53 Fixed Dose Formulations
02 Excipients
02 Antibiotics
- 15 New Herbs and Herbal Products Monographs
- 03 New Radiopharmaceutical Monographs
- 14 New Veterinary Non-Biological Monographs
- 18 New Biological Monographs :
- 02 Vaccines & Immunosera for Human Use
- 06 Biotechnology Derived Therapeutic Products
- 10 Blood and Blood Related Products
Standards for New Drugs, Drugs under National Health Programme & Drugs in National List of Essential Medicines have been included
For ease of access and to make Pharmacopoeia more user friendly index has been incorporated in Volume 1 along with that already existing in Volume IV of IP
IP 2018 contain additional new monographs, namely, Amarbel, Anise Oil, Belladona Dry Extract Tablet, Citronella Oil (Geraniol type), Citronella Oil (Java type), Green Coffee Bean Extract, Horse Chestnut Dry Extract, There is a separate chapter on general requirements – Herbs and Herbal Products. The specific monographs are mentioned in an alphabetical order. The important features with special reference to quality specifications of herbs and herbal products included a new general chapter on DNA-based authentication technique to rule
out the adulterants. [9]
Addendum 2019 contains 66 new monographs including chemical (61), herbs and herbal products (03), and radiopharmaceuticals preparations (02).
These three herbal products where added
1 Berberine Chloride
2 Henna Dry Powder
3 Karipatta [10]
Addendum 2021 to IP 2018 contains a total of 66 new drug monographs [including 59 Chemical, 05 Herbal Products, and 02 Blood-Related Products] and 04 new General Chapters. In addition, a total of 260 monograph amendments have also been included in the content of the IP Addendum 2021 that would further upgrade the quality of drug standards included in the IP.
These herbal monograph where added in 2021 Addendum
1 Bael Fruit
2 Chauliai
3 Patho
4 Upakunchika
The effective date of this Addendum has been kept as 1st October 2021 and a total period of six months is being given to the stakeholders to implement the new standards included therein.[11]
Quality aspect, Safety aspect, Efficacy aspect
Herbal drugs obtained from medicinal plants are used by a majority of the people because of their safety and less side effects, but it is not completely true that herbal products do not have any side effects or toxic effects, they do carry risks. Regulatory authorities of different countries
regulate the quality and standard of herbal drugs on the basis of problems associated with them such as herb-drug interaction, side effects, toxicity and adverse effects. The International Drug Monitoring Program of World Health Organization (WHO) has made certain guidelines for herbal drugs evaluation and quality control analysis. The WHO has done various efforts for the improvement of herbal drugs in the context of their safety and efficacy. The herbal drug toxicity arises when the drug is used without proper indications, in large doses, or with other drugs, for longer duration without consultation of a physician, and manufactured appropriately.
Objectives: This mini review has been written to discuss the current status of regulatory and quality aspects of herbal drugs. The regulatory guidelines of herbal drugs need to be improved along with the Standard Operating Procedures (SOPs) and Good Manufacturing Practices (GMP) guidelines.[17]
Regulatory aspect: Standardization of herbal drugs and their formulations, stability and safety is a basic requirement for creating confidence in public about their therapeutic use. Globally, herbal drugs regulations exist in many developed countries like USA, Brazil, Australia, Canada, Germany and developing countries such as India, Indonesia and Bangladesh; however, many countries still lack a regulatory mechanism. However, the regulatory mechanisms for the manufacturing and marketing authorization are not uniform in different countries. In India, herbal medicines are regulated as per the first schedule to the Drugs and Cosmetics Act 1940 and Rules 1945 thereunder. Schedule T, Rule 157 under the Drugs and Cosmetics Rules, 1945 specifies the requirements about the good manufacturing practices for Ayurvedic, Siddha and Unani Medicines. The Central Drugs Standards Control Organization and IPC are making continuous efforts for approval and setting the standards of medicines, respectively (Kalaiselvan, Prakash, Kalaivani, et al., 2014; Prakash, Pandey, Gupta et al., 2016).
The Central Government after consultation with the Drugs Technical Advisory Board has notified Drugs and Cosmetics Rules, 2015 to amend the Drugs and Cosmetics Rules, 1945 vide GSR 918(E) (2015) dated 30 November 2015. The rule describes the regulatory requirements for manufacturing/marketing of phytopharmaceutical drugs. The central and state drug regulatory authorities are entrusted to enforce these regulations.
National Policy on Indian Systems of Medicine and Homoeopathy – 2002 (2002) states … a large number of units exist in large, medium, small and tiny sectors. The safety, efficacy, quality of drugs and their rational use have not been assured. Though enforcement mechanism has been envisaged in the Act, and is also in place in most of the States, implementation of the enforcement laws leaves much to be desired. There is reluctance on the part of a large number of manufacturers to adhere to good manufacturing practices. Preparation of formularies and pharmacopoeial standards have been speeded up but a lot is yet to be completed. There is no assurance whatsoever that Formularies and Pharmacopoeial standards are being followed by the Indian Systems of Medicine & Homoeopathy drug manufacturers.
National Health Policy (2017) recognizes the need to standardize and validate Ayurvedic medicines and establish a robust and effective quality control mechanism for AYUSH drugs. The policy advocates strengthening and rationalizing the drug regulatory system, promotion of research and development in the pharmaceutical sector and building synergy and evolving a convergent approach with related sectors. The IP serves as a useful regulatory document for the Indian Drug Regulatory Authorities for the manufacture, marketing and inspection of quality of medicines including herbs and herbal products.
Stability testing of herbal drugs:
Stability of herbal drugs and their formulations is an important criteria during their development process as well as after the marketing authorization. Critical systematic reviews and meta-analysis of available reports provide a reliable and rigorous method of assuring safety and efficacy of herbal drug/product. Similar to synthetic drugs in modern system of medicines, herbal products are also required to be subjected to comprehensive systematic testing in order to establish and ensure consistent therapeutic efficacy and safety throughout their shelf lives. The drug regulatory agencies across the globe such as European Medicines Agency, US Food and Drug Administration have recommended guidelines for conducting stability studies on herbal drugs and products and dossier requirement of stability data submission for product registration. The WHO (2006) has also recommended for stability studies on herbal drugs and finished herbal formulations. The stability testing of herbal drugs, challenges, regulatory compliance and perspectives has been recently reviewed (Bansal, Suthar, Kaur, et al., 2016). In India, for a phytopharmaceutical drug, there is a provision in Drugs and Cosmetics Rules, 1945 (notified vide GSR 918(E) (2015), Ministry of Health and Family Welfare) for requirement of stability data.
Reference standard
The pharmacopoeias may describe the use of reference standards in the analysis of individual herbal medicines. RS may be authentic pure compounds or extracts of herbal materials or powdered herbal materials used for comparison. The RS established by individual pharmacopoeias are suitable for testing purposes
Harmonization of standards The standards set out in IP are harmonized with prevailing international trend and keeping in mind the Indian context. The analytical methods feasibility, consensus of experts, inputs of stakeholders on the draft monographs and an all transparent approach is adopted during the process of standards setting. WHO has identified the need for harmonization of herbal monographs, and accordingly, necessary steps are underway for developing good pharmacopoeial practices guidelines for herbal monographs, and IP is the lead pharmacopoeia (WHO, 2015).
Herbs and herbal products such as herbal extract and herbal formulation:
Herbal dosage forms are the physical form (liquid, solid, semi solid) of herbal products produced from herbs, with or without excipients, in a particular formulation (such as decoctions, tablets and ointments). They are produced either from herbal materials (such as dried roots or fresh juices) or herbal preparations (such as extracts).
Herbal medicines include herbs and/or herbal materials and/or herbal preparations and/or finished herbal products in a form suitable for administration to patients.
Note: In some countries herbal medicines may contain, by tradition, natural organic or inorganic active ingredients that are not of plant origin (e.g. animal and mineral materials, fungi, algae or lichens).
Herbs include crude plant materials such as leaves, flowers, fruit, seed, stems, wood, bark, roots, rhizomes or other plant parts, which may be entire, fragmented or powdered.
Herbal materials include, in addition to herbs, fresh juices, gums, fixed oils, essential oils, resins and dry powders of herbs. In some countries these materials may be processed by various local procedures, such as steaming, roasting or stir-baking with honey, alcoholic beverages or other plant materials.
Herbal preparations are the basis for finished herbal products and may include comminuted or powdered herbal materials, or extracts, tinctures and fatty oils of herbal materials. They are produced by extraction, fractionation, purification, concentration or other physical or biological processes. They also include preparations made by steeping or heating herbal materials in alcoholic beverages and/or honey or in other materials.
Finished herbal products consist of one or more herbal preparations made from one or more herbs (i.e. from different herbal preparations made from the same plant as well as herbal preparations from different plants. Products containing different plant materials are called “mixture herbal products”).
Finished herbal products and mixture herbal products may contain excipients in addition to the active ingredients. However, finished products or mixture herbal products to which chemically defined active substances have been added, including synthetic compounds and/ or isolated constituents from herbal materials, are not considered to be “herbal”.
Medicinal plant materials: see Herbal materials Medicinal plants are plants (wild or cultivated) used for medicinal purposes. State of the herbal material means whole, fragmented, peeled, cut, fresh or dried
Content of individual herbal monograph
Monograph: Each monograph begins with a definition and introductory paragraph indicating the formulation composition, scientific names of the drugs used with their botanical parts along with a brief account of the method of preparation.
The requirements given in the monographs are not framed to provide against all impurities, contaminants or adulterants; they provide appropriate limits only for possible impurities that may be permitted to a certain extent. Material found to contain an impurity, contaminant or adulterant which is not detectable by means of the prescribed tests are also to be considered as impurity should rational consideration require its absence.
Title: Should include latin binomial nomenclature, synonyms and vernacular names.
Definition: The definition provides details about the subject of the monograph and includes the Latin binomial name and the taxonomic authority (abbreviation if used should be according to internationally-accepted rules), the plant family name if required by national legislations, the well-established common local name and English common name (if available, in addition to the scientific name), and well-recognized synonyms, the plant part(s) (i.e. aerial parts, root, leaves, flowers, rhizome, etc.), plant material (e.g. resin, gum-resin and where applicable, its state and type of herbal preparation (e.g. liquid extract, dry extract) and its dosage form (tablet, capsule, etc.). When necessary, as dictated and supported by data, the definition also states the season or period in which plant material should be harvested according to Good Agricultural and Collection Practices (GACP). If more than one species is included in the monograph the definition should include, for each of the species, the requirements listed above. The definition should include the chemical names, and/or molecular formulas of relevant known constituents, for which there is a specified range or minimum content, in percentage, usually calculated on the basis of the dry weight of the herbal medicines. Where a monograph applies to the herbal medicines in different states or stages of processing (DEFINE “STATES” IN GLOSSARY, including…), this is stated in the definition.[1]
Description: Statement given under this title is not to be interpreted in a strict sense although they may help in the evaluation of an article. However substantial departure form the requirement will not be acceptable.
Geographical distribution:
The region is estimated to have approximately 1,700 known medicinal plant species. Some of the sought after species of these regions are Aconitum heterophyllum Wall. Ex Royle, Ferula jaeschkeana Vatke and Saussurea costus (Balc.) Lipsd. S. costus is in fact confined to only the Himalayan region of Jammu and Kashmir. These critically endangered wild varieties are enlisted in the Convention on International Trade in Endangered Species of flora and fauna (CITES).
Trans Himalaya
The section is estimated to harbour approximately 700 known medicinal plants. Some of the well known ones, existing in very cold and desert like conditions of the region are Ephedra gerardiana Wall., Hippophae rhamnoides L. and Arnebia euchroma (Royle) John etc.
Central and Eastern Himalaya
The biotic provinces, put together, are estimated to harbour around 1,200 known medicinal plant species. A few of the well known plants are Nardostachys grandiflora DC., Taxus wallichiana Zucc. (listed in CITES), Rhododendron anthopogon D.Don and Panax pseudoginseng Wall (only found in the Eastern Himalayas).
North east
The region with an estimated 2000 medicinal plant species is yet another high biodiversity region of the country. This zone is amongst one of the 18 hot spots, which presents a high level of endemism. It consists of two biotic provinces namely Brahmaputra Valley and Assam Hills. Aquilaria malaccensis Lam.(listed in CITES), Smilax glabra Roxb., Ambroma augusta (L.) L.f. and Hydnocarpus kurzii (King) Warb are some of the well known medicinal plants found here.
Desert
The biogeographic zone consisting of Kutch and Thar biotic provinces is a haven for almost 500 known medicinal plant species. Some of the harvested plants of this region are Convolvulus microphyllus Seib ex Spreng (Syn C. pluricaulis Chois), Tecomella undulata (Sm.) Seem, Citrullus colocynthis (L.) Schrader and Cressa cretica L.
Deccan Peninsula
It covers the largest chunk of landmass amongst all the 10 biogeographic zones and consists of five biotic provinces – South Deccan Plateau, Central Plateau, Eastern Plateau, Chhotanagpur and Central Highlands. With a total number of known species estimated to be around 3000, the endemic species here are Pterocarpus santalinus L.f., Decalepis hamiltonii Wight & Arn.,
Terminalia pallida Brandis and Shorea tumbuggaia Roxb. of which Pterocarpus santalinus L.f. is enlisted in CITES.
Gangetic Plain
Upper Gangetic Plain and Lower Gangetic Plain biotic provinces is estimated to have around 1000 known medicinal plant species. A few of the well known species found in the region are Holarrhena pubescens (Buch-Ham.) Wallich ex A.DC., Mallotus philippensis (Lam.) Muell.-Arg., Pluchea lanceolata C. B.Clarke and Peganum harmala L.
Semi arid zone
It consists of biotic provinces of Punjab and Gujarat-Rajwar and is estimated to be home to around 1,000 known medicinal plant species. Commiphora wightii (A.) Bhandari, Caesalpinia bonduc (L.) Roxb., Balanites aegyptiaca (L.) Delile and Tribulus rajasthanensis.
West and East Coast
The coastal biogeographic zone is estimated to harbour around 500 plant species of known medicinal plants. A few of the well known ones are Rhizophora mucronata Lam., Acanthus ilicifolius L., Avicennia marina (forsk.) Vierh and Sonneratia caseolaris (L.) Engl.
Andaman, Nicobar and Lakshadweep
Some of the important medicinal plants of this island biogeographic zone, which is estimated to have around 1000 species consists of Calophyllum inophyllum L., Adenanthera pavonina L., Barringtonia asiatica (L.) Kurz and Aesandra butyracea (Roxb.) Baehni.
Western Ghats
The biogeographic zone consisting of Malabar Coast and the Ghats is also one of the 18 biodiversity hot spots recognised across the globe with nearly 2000 medicinal plants. Endemic species in this region are Myristica malabarica Lam., Garcinia indica (Dup.) Choisy, Utleria salicifolia Bedd. ex.Hook.f. and Vateria indica L. These species have been assessed to be under varying degree of threat of extinction ranging from vulnerable (VU) in case of Vateria indica L. to critically endangered (CR) for Utleria salicifolia Bedd. ex.Hook.f.[20]
Name of the Formulation: The name given on top of each monograph is in Sanskrit, as mentioned in the Ayurvedic Formulary of India (AFI) and will be considered official. These names have been arranged in English alphabetical order under each category of dosage form.
Standards: For statutory purposes, the following shall be considered official standards: Definition, Formulation composition, Identification, Physico-chemical parameters, Assay and Other requirements.
Added Substances: A formulation contains no added substances except when specifically permitted in the individual monograph. Unless otherwise specified in the individual monograph, or elsewhere in the General Notices, suitable substances may be added from the approved list of Drugs and Cosmetics Rules, under Rule 169 to a formulation to enhance its stability, usefulness, elegance, or to facilitate its preparation. Such auxiliary substances shall be harmless in the amounts used, shall not exceed the minimum quantity required to provide their intended effect, shall not impair the therapeutic efficacy or the bioavailability and safety of the preparation and shall not interfere with the tests and assays prescribed for determining compliance with the official standards. Particular care should be taken to ensure that such substances are free from harmful organisms. Though the manufacturer of a formulation is given the freedom to use an added substance, the manufacturer must guarantee the innocuousness of the added substance. The manufacturer shall also be responsible to explain to the appropriate authority, if needed, regarding the purpose of the added substance(s).
Odour and Taste: Wherever a specific odour has been observed it has been mentioned as characteristic for that formulation, but the description as ‘odourless’ or ‘no odour’ has generally been avoided in the Description where a substance has no odour. Where a characteristic odour is said to be present it is examined by smelling the drug directly after opening the container. If such an odour is discernible, the contents are rapidly transferred to an open vessel and reexamined after 15 minutes. If odour persists to be discernible, the sample complies with the description for odour, characteristic for that formulation.
The taste of a drug is examined by taking a small quantity of drug by the tip of moist glass rod and allowing it on tongue previously moistened with water. This does not apply in the case of poisonous drugs.
Identity, Purity and Strength:
Under the heading “Identification”, tests are provided as an aid to identification and are described in the respective monographs and included.
Herbal/Plant drugs should be duly identified and authenticated and should be free from insects, pests, fungi, microorganisms, pesticides, and other animal matter including animal excreta, should be within the permitted and specified limits for lead, arsenic and heavy metals, and show no abnormal odour, colour, sliminess, mould or any sign of deterioration. Herbal/plant drugs should be duly identified and authenticated and should be free from insects, pests and other animal matter including animal excreta, should have, within the permitted and specified limits, for fungi, microorganisms, pesticides, and heavy metals, and show no abnormal odour, colour, sliminess, mould or any sign of deterioration.
Quantitative tests like total ash, acid-insoluble ash, water-soluble ash, alcohol-soluble extractive, water-soluble extractive, moisture content, volatile oil content and assays are the parameters upon which the standards of Pharmacopoeia depend. Except for Assays, which are covered under each monograph, the methods of determination for the others are given in Appendices IP
Thin-Layer Chromatography (TLC): Under this title, the Rf values given in the monographs are not absolute but only indicative. The analyst may use any other solvent system and detecting reagent to establish the identity of any particular chemical constituent reported to be present in the test substance. However, in case of dispute the pharmacopoeial method would prevail. Unless specified in the individual monograph all TLC have been carried out on pre-coated Silica gel 60F254 aluminium plates
Therapeutic uses: Therapeutic uses wherever given are as mentioned in the API.
Doses: The doses mentioned in the monograph are in the metric system, which are approximate conversions from classical weights mentioned in Ayurvedic textsLabeling: In general, the labeling of drugs and pharmaceuticals is governed by the Drugs and Cosmetics Act, 1940 and Rules there under.
Individual Monographs on Herbal drug
Honey

Madhu, Honey Purified, Mel, Shaed
Honey is the sugar secretion deposited in honey comb by the bees, Apis mellifera, Apis dorsata and other species of Apis, belonging to family Apidae, order Hymenoptera.
Category: Demulcent and Sweetening agent
Description :
Colour – Pale yellow or yellowish-brown liquid
Odour – Characteristics
Taste – Sweet and faintly aicd
Identification:
A To an aqueous solution of honey (2 ml) Fehlings solution 1 and 2 are added and the reaction mixture is heated on steam bath for 5-10 minutes, a brick red colour is produced due to presence of reducing sugars.
2. Fiehe’s test: Honey is shaken with petroleum ether (5 ml) for 5-10 minutes. The upper ethereal layer is separated and evaporated in a china dish. On addition of 1% of resorcinol in HCI (1 ml) a transient red colour is formed in natural honey while in adulterated honey, the colour persists for some time.
Standards of Quality
Weight per ml – 1.35 – 1.36 g
Specific rotation – +3°- -10°
Total ash – 0.1-0.8 per cent
It is soluble in water, but insoluble in alcohol.
It has to pass limit tests for chloride and sulphate
It is syrupy thick liquid, translucent when fresh and on keeping it becomes opaque and granular due to the crystallisation of glucose.
Chemical Content
Honey is an aqueous solution of glucose 35 per cent (±3 per cent), fructose 45 per cent (±5 per cent ) and sucrose about 2 per cent. The proportion of sugar may vary depending upon the source of necter and the enzymatic activity responsible for converting necter into the honey. The other constituents of honey are maltose, gum, traces of succinic acid, acetic acid, dextrin, formic acid, colouring matters, enzyme and trace of vitamins. Proteins and pollen grains from various flowers are also found in honey.
Storage: keep it in a cool location away from direct sunlight and in a tightly sealed container
REFERENCES
1 Ayurvedic Pharmacopoeia of India Part – I Volume – I Government of India Ministry of Health and Family Welfare Department of Ayush Page No vii, ix.
2. European Union herbal monograph on Curcuma longa L., rhizome EMA/HMPC/329755/2017
3 . Indian Pharmacopoeia 2007a. (5th edn). The Indian Pharmacopoeia Commission, Ghaziabad.
4. Indian Pharmacopoeia 2010a. (6th edn). The Indian Pharmacopoeia Commission, Ghaziabad.
5. Indian Pharmacopoeia 2010b, Addendum 2012. The Indian Pharmacopoeia Commission, Ghaziabad.
6. Indian Pharmacopoeia 2014a. (7th edn). The Indian Pharmacopoeia Commission, Ghaziabad.
7. Indian Pharmacopoeia 2014b, Addendum 2015. The Indian Pharmacopoeia Commission, Ghaziabad.
8. Indian Pharmacopoeia 2014c, Addendum 2016. The Indian Pharmacopoeia Commission, Ghaziabad.
9. Indian Pharmacopoeia 2018a. (8th edn). The Indian Pharmacopoeia Commission, Ghaziabad
10. Indian Pharmacopoeia 2018b, Addendum 2019. The Indian Pharmacopoeia Commission, Ghaziabad.
11. Indian Pharmacopoeia 2018c, Addendum 2021. The Indian Pharmacopoeia Commission, Ghaziabad.
12. PHYTOTHERAPY RESEARCH Phytother. Res. (2017) Published online in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/ptr.5933
13. NHP 2017. http://cdsco.nic.in/writereaddata/National-Health-Policy.pdf
14. NMPB, 2017. Available from (http://www.nmpb.nic.in/) Accessed on 30.06.2017
16. WHO, 2003. Available from http://www.who.int/mediacentre/factsheets/2003/fs134/en/. (accessed on 10.07.17) World Health Organization Good Practices for Pharmaceutical
17. Quality Control Laboratories. In: Quality Control Methods for Herbal Drugs 2011, 135–175. Printed in Malta. World Health Organization. 2004. The World Medicines Situation. World Health Organization: Geneva.
18. World Health Organization. 2006. Supplementary guidelines on good manufacturing practices for the manufacture of herbal medicines. (WHO Technical Series, No.953) World Health Organisation, Geneva.
19. World Health Organization. GOOD PHARMACOPOEIAL PRACTICES Draft chapter on monographs on herbal medicines during the 8th international meeting of world pharmacopoeias, terminology added 7/9/2017)
21. Text book of Pharmacognosy by C.K. Kokate, Purohit, Gokhlae 54th Edition, Nirali Prakashan, New Delhi. Page No. 8.43
Preparation of Herbal Monograph
Also Read
1 Honey Synonyms Biological Source, Chemical Constituents, Uses